A phase 2b study to evaluate the safety and efficacy of VRC01 broadly neutralizing monoclonal antibody in reducing acquisition of HIV-1 infection in women in sub-Saharan Africa.

JANUARY 2021: AMP EFFICACY STUDY RESULTS RELEASED
What is HVTN 703/HPTN 081?
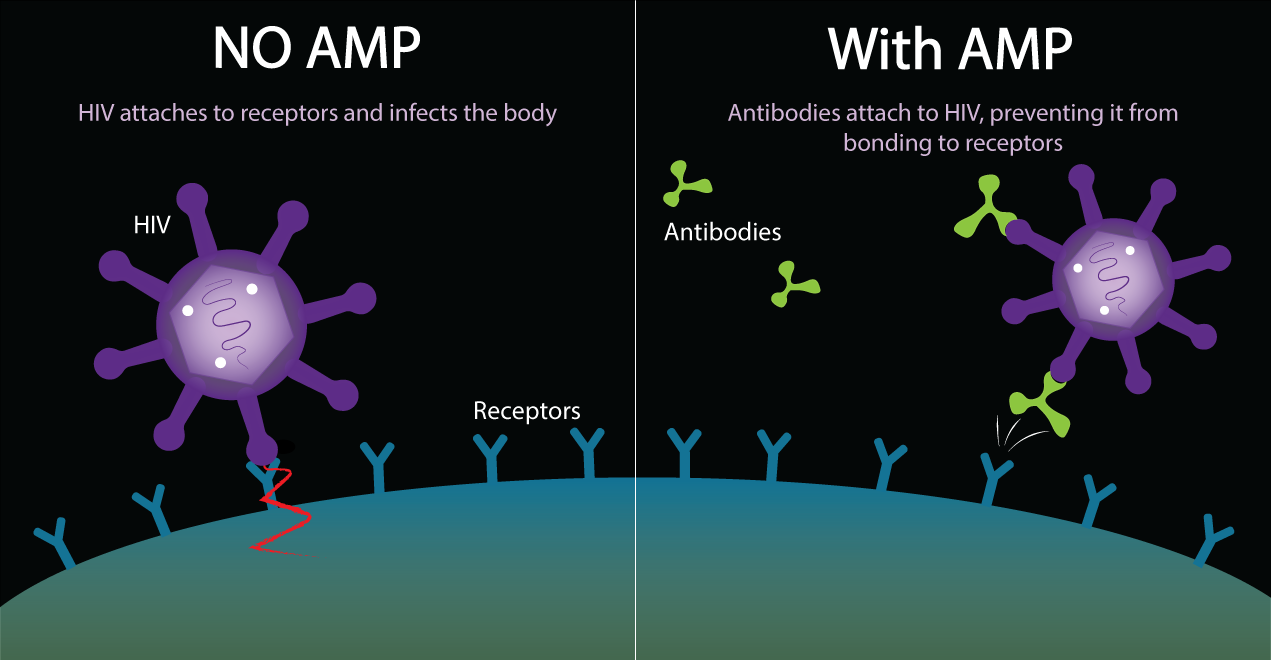 The AMP Study (also known as HVTN 703/HPTN 081) tests an experimental antibody against HIV. AMP stands for Antibody Mediated Prevention. This is the idea of giving people antibodies that fight HIV to see if they will protect people from acquiring HIV.
The AMP Study (also known as HVTN 703/HPTN 081) tests an experimental antibody against HIV. AMP stands for Antibody Mediated Prevention. This is the idea of giving people antibodies that fight HIV to see if they will protect people from acquiring HIV.
The AMP study tests an antibody called VRC01, a manufactured antibody against HIV. This is a new idea for HIV prevention that is related to what has been done in HIV vaccine research. In traditional HIV vaccine studies, people get a vaccine and researchers wait to see if their bodies will make antibodies against HIV in response to the vaccine. In this study, we will skip that step, and give people the antibodies directly.
 Who is participating in the study?
Who is participating in the study?
The study will enroll and follow about 1,900 adult women in sub-Saharan Africa who are behaviorally vulnerable to HIV. To join this study, a woman must be a healthy adult and not living with HIV. She cannot be pregnant or breastfeeding. There are also other criteria that must be met. We will ask women about their medical history, give them a physical exam, and take blood and urine samples for testing. We will also ask women about their recent sexual activity and drug use.
Why is HVTN 703/HPTN 081 important?
Women are among those at highest risk of HIV infection in Africa. They have the greatest need for new tools to prevent HIV infection, and it is important to include them in studies looking at new prevention methods.
All HVTN and HPTN studies work toward our shared mission of finding effective ways to prevent HIV infection. The main purposes of the study are to:
* Gather more information about the safety of VRC01;
* See if people can receive the antibody without becoming too uncomfortable;
* Test whether the VRC01 antibody can prevent HIV infection in people;
* Find out how much VRC01 is needed for protection, if we find that it does work to prevent HIV infection.
What happens during the study?
VRC01 will be given using intravenous infusions. This is more commonly known as getting an IV, or getting a drip. The IV is given to the study participant every eight weeks for 30-60 minutes. To get an IV, a sterile needle is used to place a small plastic tube into a vein in the participant’s arm. A bag of fluid is hung from a pole and connected to a pump, which controls how quickly the contents of the bag flow through the tube into the participant’s arm.
There will be 3 different groups in this study. One third of study participants will get a higher dose of the antibody in their IV. One third will get a lower dose of the antibody in their IV. One third will get an infusion of sterile salt water without any antibody in it. This is called a placebo. Participants will be enrolled in the study for about two years.
What were the results of the study?
The proof-of-concept AMP studies demonstrated that a broadly neutralizing antibody (bnAb) called VRC01 was effective at preventing the acquisition of HIV strains that were sensitive to the bnAb. This was assessed by a laboratory test that measures a virus’ susceptibility to neutralization by an antibody. Read more.
➤ For study-specific materials, please log into the member portal on the HVTN website.
HIV Prevention & The AMP Study
Study Documents
HVTN 703/HPTN 081 Version 4.0
- HVTN 703/HPTN 081 Protocol V4.0 – 14 December 2017
- HVTN 703/HPTN 081 Full Protocol Amendment #3 – 14 December 2017
HVTN 703/HPTN 081 Version 3.0
- HVTN 703/HPTN 081 Protocol V3.0 – 25 August 2017
- HVTN 703/HPTN 081 Full Protocol Amendment #2 – 25 August 2017
HVTN 703/HPTN 081 Version 2.0
HVTN 703/HPTN 081 Version 1.0
Presentations
- The HIV Prevention Toolbox: More Tools Needed / Mike Chirenje - August 2016
- Introduction to the Science of HVTN 703/HPTN 081 / Nyaradzo Mgodi - August 2016
- Recruiting, Retaining and Continued Engagement of Women At-Risk for HIV Infection / Portia Hunidzarira - August 2016
More Study Documents
Click here to request access to more documents in Microsoft TEAMS.
You will need a Microsoft account to log in to TEAMS. Click here for instructions on setting up your Microsoft account and accessing the TEAMS platform.
Study Details
A phase 2b study to evaluate the safety and efficacy of VRC01 broadly neutralizing monoclonal antibody in reducing acquisition of HIV-1 infection in women in sub-Saharan Africa.
Multicenter, randomized, controlled, double-blind trial.
Women in sub-Saharan Africa aged 18-40 who are at risk of HIV-1 infection.
1900 women in sub-Saharan Africa aged 18-40 who are at risk of HIV-1 infection; 2:1 active:control allocation; total approximately 1268 VRC01 mAb, 634 control
24 months of scheduled clinic visits; 60 months total study duration (includes enrollment and follow-up, including follow-up for HIV-infected participants).
VRC01 mAb by IV infusion at a dose of 10 mg/kg or 30 mg/kg every 8 weeks, or to receive placebo infusions every 8 weeks.
1. To evaluate the safety and tolerability of VRC01 mAb administered through IV infusion in sub-Saharan African women.
2. To determine if the VRC01 mAb prevents HIV-1 infection and to estimate the level of efficacy in sub-Saharan African women.
To develop a marker(s) of the VRC01 mAb that correlates with the level and antigenic specificity of protection against HIV-1 infection and to provide insight into the mechanistic correlates of protection.

