Educational Resources
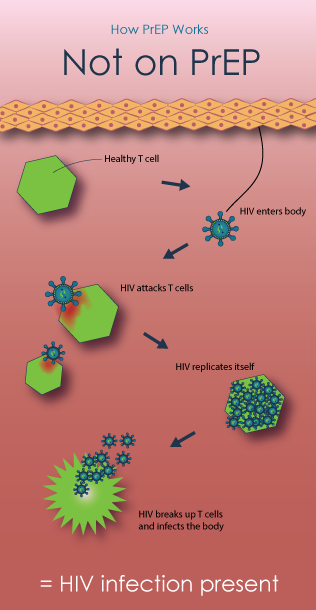
HIV, or human immunodeficiency virus, attacks and destroys the immune system. If left untreated, it can result in acquired immunodeficiency syndrome, or AIDS, the most advanced stage of HIV infection. Unlike other viruses, HIV never leaves the body completely, therefore once acquired, you have it for the rest of your life. Currently, there is no cure for HIV; however, there are treatments available to help control the virus, prolong life, and reduce the chance of transmitting it to others.
HIV attacks CD4 cells (T cells), which help the immune system fight infections. Left untreated, HIV reduces the number of T cells in the body, increasing the likelihood of infections and certain cancers. Over time, HIV can destroy so many T cells, the body is left unable to fight off any infections or diseases.
HIV is transmitted through contact with certain body fluids, such as blood, semen, pre-seminal fluid, vaginal fluids, rectal fluids, and breast milk. Exposure to these fluids can happen during unprotected sex, sharing needles, or mothers passing the virus to their children during pregnancy, birth, and breastfeeding. You cannot acquire HIV through contact with a person living with HIV’s sweat, tears, saliva, bath or pool water, toilet seats, doorknobs, or by sharing dishes or drinking glasses, hugging, or shaking hands. Also, HIV does not spread through the air or insect bites such as mosquitos and ticks.
Flu-like symptoms, including fever, headache, or rash, are common within a few weeks of HIV acquisition. These symptoms may come and go for several weeks or last for days or weeks.
Blood and saliva tests are used to diagnose HIV. These tests look for antibodies the body creates to fight the virus. Therefore, it is important to get tested as soon as you suspect exposure to HIV.
Antiretroviral therapy, or ART, is recommended for anyone living with HIV. ART involves taking a daily combination of HIV medicines to reduce HIV levels in the body (i.e., viral load). While HIV medicines cannot cure the virus, they can help prevent it from advancing to AIDS. HIV medicines also help reduce the risk of transmission to others. People living with HIV with undetectable viral loads (i.e., no longer detected by blood tests) cannot transmit the virus to others.
ART for people living with HIV helps prevent transmission. For people at high risk for acquisition of HIV, pre-exposure prophylaxis (PrEP) and post-exposure prophylaxis (PEP) is recommended. Other prevention steps include avoiding sexually transmitted diseases, using protection during sex (e.g., condoms, dental dams), avoid sharing or reusing needles, and seeking help with substance abuse and mental health issues.
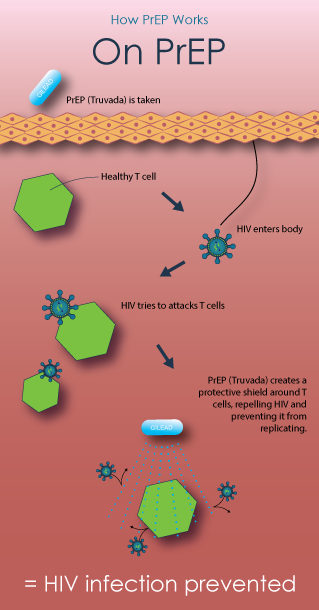
Pre-exposure prophylaxis, or PrEP, for HIV prevention is a strategy that involves use of antiretroviral medications (ARVs) to reduce the risk of HIV infection in people who are HIV-negative. All of the current PrEP trials are testing tenofovir-based regimens—using either TDF/FTC (an antiretroviral containing tenofovir (TDF) and emtricitabine (FTC), sold under the brand name Truvada) or TDF (an antiretroviral pill marketed under the brand name Viread). Learn more from AVAC.
 |  |
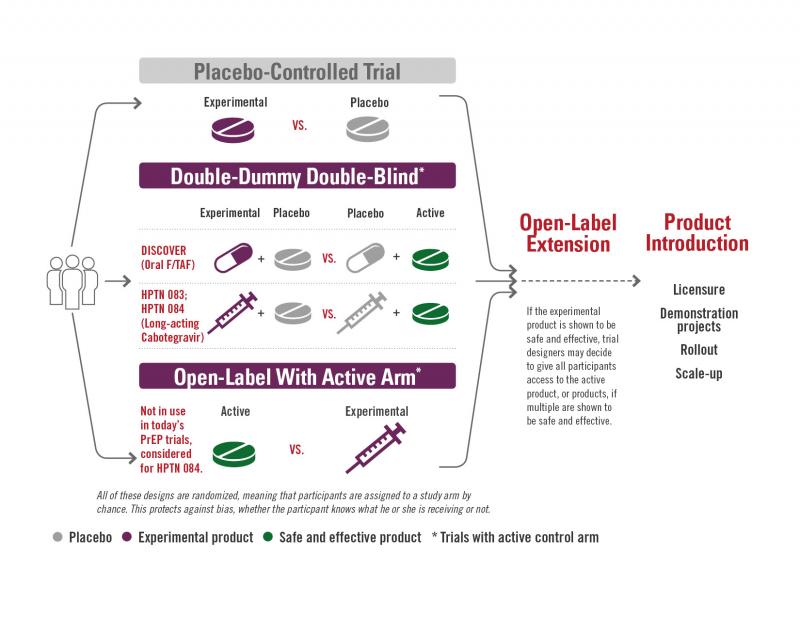
A clinical study involves either observational research or research that studies medical, surgical, or behavioral interventions in people, often called a clinical trial. They are the primary way researchers conclude if a new treatment, like a new drug, is safe and effective in people.
In the HPTN’s HIV prevention clinical studies, we examine use of antiretroviral drugs (antiretroviral therapy and pre-exposure prophylaxis); interventions for substance abuse, particularly injection drug use; behavioral risk reduction interventions, and structural interventions.
There are four phases of clinical studies:
Phase 1 Clinical Studies
Phase I studies test the safety of a product. This means seeing if there are side effects, and whether the human body can tolerate the experimental product, such as a new drug. This product is often compared to a group that receives a placebo, a substance such as sterile saltwater that has no active ingredients. These studies are conducted with a small group of people (usually less than 100) and typically last 12 to 18 months.
Phase 2 Clinical Studies
Phase 2 studies look at questions such as the maximum tolerated dose of a product, the optimal schedule for giving the product (how many doses, and at what time intervals), and whether the immune system is having the desired responses. These responses can include the production of antibodies, the production of T cells, and other immune system markers. These studies are conducted with a medium-size population of volunteers (usually a few hundred to 1,000) and the studies can last up to two years.
Phase 2b Clinical Studies
Phase 2b studies are a way to get an early look at whether the product is effective at preventing infection or disease. They are sometimes known as Proof of Concept or Test of Concept studies. A group of several thousand people who are at increased risk for infection or disease are enrolled, and the studies can last about 2-5 years. Based on the data from these studies, researchers can see whether the results seem favorable, supporting moving ahead to Phase 3. If the results are less favorable, it means that researchers can redirect their efforts and save the expenses of large Phase 3 studies.
Phase 3 Clinical Studies
Phase 3 studies are where researchers can ask the questions, “Does this product prevent new infections?” Or “if people do become infected, does the product help them control the infection so that it doesn’t become severe disease?” These studies involve many thousands of people, usually including some participants who are at increased risk for infection.
Phase 4 Clinical Studies
Phase 4 studies may take place after a product has been found to be effective in some populations and are utilized to gather additional information about safety and effectiveness by testing the product in additional populations such as children or pregnant women. They are sometimes known as bridging studies, or post-licensure studies. These studies may be conducted in anyone seeking a prevention option from their physician.
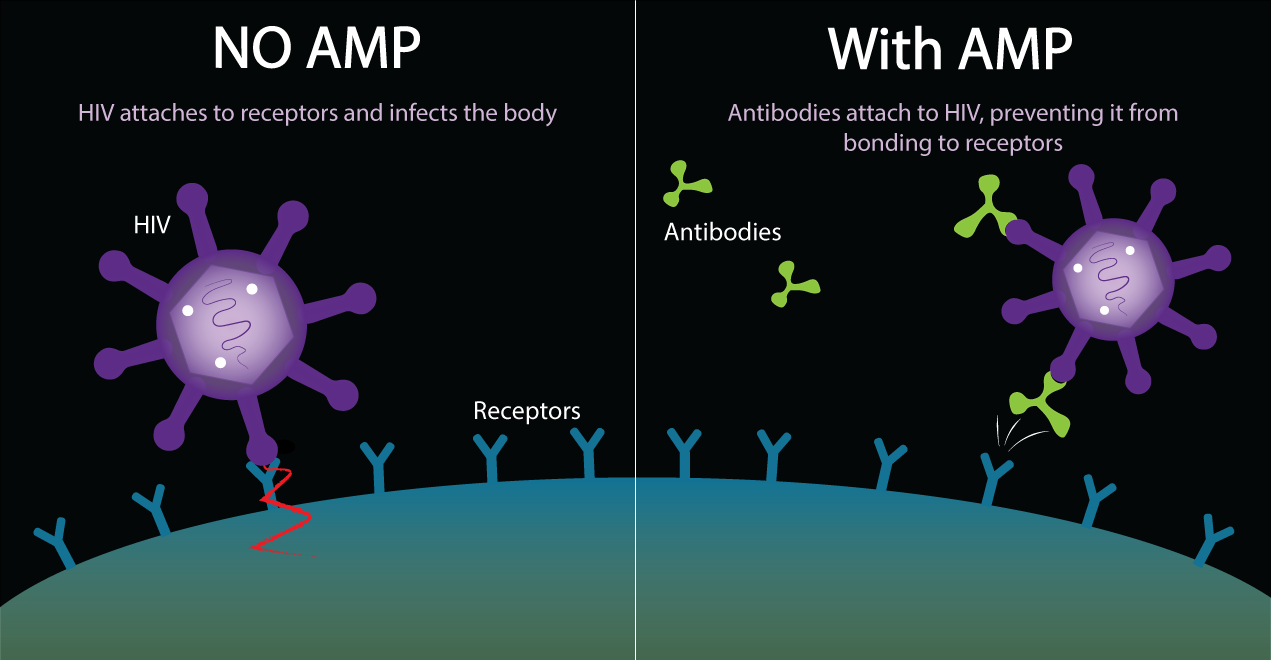
AMP stands for Antibody Mediated Prevention. This is the idea of giving people antibodies to see if they will protect people from acquiring HIV. This antibody is a broadly neutralizing antibody to HIV. Its name is VRC01. It stops HIV from binding to human T-cells by attaching to the virus and preventing it from infecting the T-cell. The VRC01 antibody is able to bind onto HIV at the CD4 binding site on the gp120 protein. This neutralizes HIV and prevents HIV from being able to attach to cells and infect them.
Learn more about our AMP studies in collaboration with the HIV Vaccine Trials Network: