HPTN 074
Integrated Treatment and Prevention for People Who Inject Drugs: A Vanguard study for a Network-based Randomized HIV Prevention Trial Comparing an Integrated Intervention Including Supported Antiretroviral Therapy to the Standard of Care
- Study Summary
- Details
- Documents
- Personnel
- Sites
- Publications
What is HPTN 074?
HPTN 074 aimed to determine the feasibility of a future trial that would assess whether an integrated intervention combining psychosocial counseling and supported referrals for antiretroviral therapy (ART) at any CD4 cell count and substance use treatment for people living with HIV who inject drugs would reduce HIV transmission to HIV-uninfected injection partners, as compared to routine care dictated by national guidelines for people living with HIV who inject drugs.
Who participated in the study?
Overall, 502 people living with HIV and 806 people with whom they injected drugs entered the study over 15 months and followed for 12 to 24 months after enrollment. The median age was 35 years. Eighty-five percent of participants enrolled were men. Most of the women who participated in the study were enrolled in Ukraine.
Why is the study important?
Injection drug use is the predominant risk behavior for HIV transmission in several parts of the world. Injection drug use is a major factor underlying the HIV epidemics in Eastern Europe, the Commonwealth of Independent States, and many parts of Asia. The largest estimated populations of people who inject drugs (PWID) are in Russia, China and the U.S. Notably, Ukraine, Vietnam and Indonesia have estimated HIV prevalence among PWID above 30 percent. This persistently high incidence of HIV infection among PWID in many locations with concentrated epidemics necessitates aggressive efforts to prevent HIV transmission.
What happened during the study?
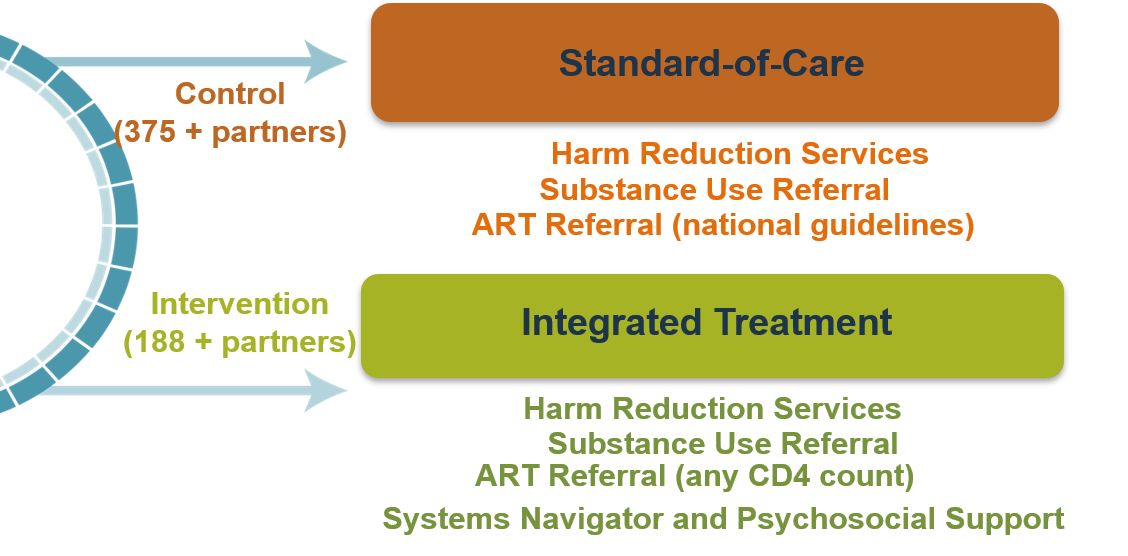
Index participants were randomized to one of two study arms. Index participants in the intervention arm received, in addition to the standard harm reduction package, an integrated intervention that included supported referrals for ART regardless of CD4 cell count and facilitated referral for substance use treatment. The psychosocial component of the integrated intervention was designed to improve engagement and retention in HIV care and substance use treatment, and included systems navigation, counseling to encourage engagement in care and adherence, and social support. Index participants in the standard of care arm received referrals for the in-country standard of care for ART and substance use treatment and a standardized harm reduction package. Network injection partners in both arms received a standardized harm reduction package with a referral for substance use treatment, consistent with national guidelines.
Study Design
What were the key study findings?
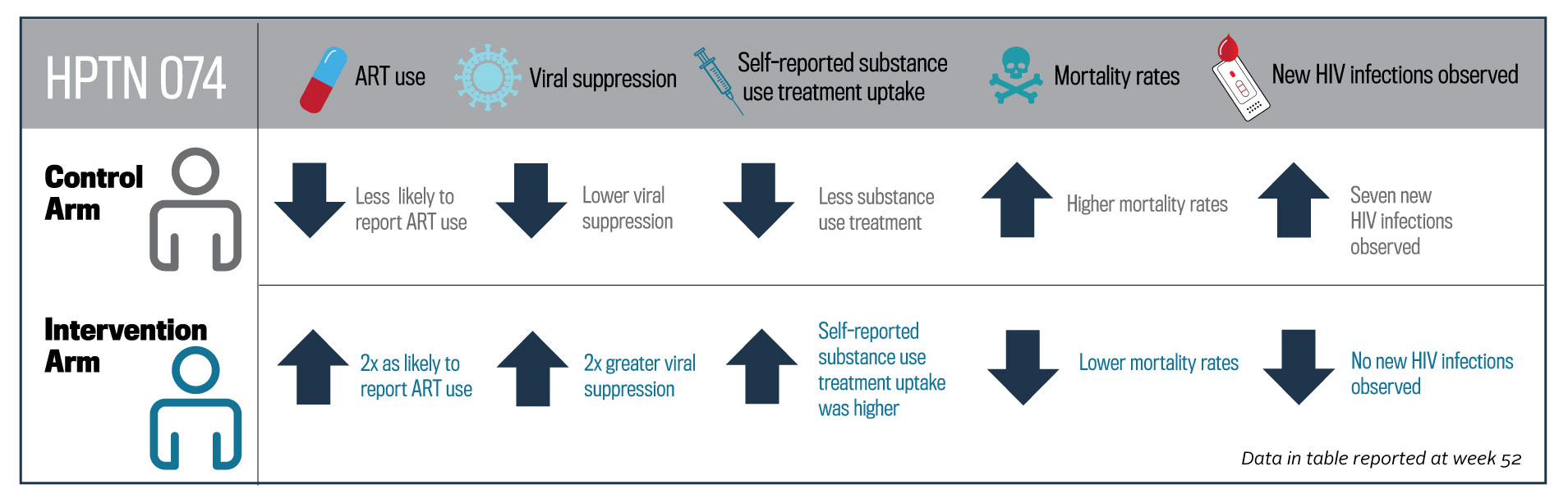
At week 26, intervention arm participants were twice as likely to report antiretroviral therapy use compared to the standard of care arm participants and twice as likely to achieve an undetectable viral load. The effects persisted at week 52. Among intervention arm participants at week 52, self-reported substance use treatment uptake was higher compared to the standard of care arm participants. Mortality was significantly lower among intervention arm participants and their partners compared to the standard of care arm participants and their partners. For partners of intervention arm participants, no new HIV infections were observed, while seven were observed among partners in the standard of care arm.
Protocol Status
Concluded
Study Purpose
The purpose of this study is to determine the feasibility of a future trial that will assess whether an integrated intervention combining psychosocial counseling and supported referrals for antiretroviral therapy (ART) at any CD4 cell count and substance use treatment for HIV-infected people who inject drugs (PWID) will reduce HIV transmission to HIV-uninfected injection partners, as compared to routine care dictated by national guidelines for HIV-infected PWID.
Study Design
This is a multi-site, two-arm, randomized, vanguard study. Network units will consist of an HIV-infected index participant and his/her HIV-uninfected network injection partner(s). Network units will be randomized to the intervention or standard of care arms in a 1:3 ratio, stratified by site. To assess feasibility of the intervention, additional interviews will be conducted with study staff (systems navigators and counselors), clinic-based stakeholders, and some index participants at each study site.
Study Population
The study population will consist of the following participant types:
Index participants: HIV-infected PWID who have an HIV viral load >= 1,000 copies/mL at screening. This may include individuals who report that they are: (a) ART-naïve, (b) ART-exposed but currently off therapy, or c) on ART.
Network injection partners: HIV-uninfected injection partners of index participants (up to five active partners per index participant at a time).
Study Size
At least 500 network units, defined as one index participant and one network injection partner, will be recruited. The study will be performed at four study sites in three countries.
Each site will enroll approximately 167 index participants and 250 network injection partners (up to five network injection partners per index participant). At least half of the index participants enrolled at each site are expected to report that they are ART-naïve at study enrollment. Provision is made for replacement of network injection partners (late-entry HIV-uninfected network injection partners) during the study.
Study Duration
Approximately 27 months at each site, with recruitment of index participants over 15 months and follow-up for a minimum of 12 months and a maximum of 24 months, regardless of study arm. All participants will end study participation when 27 months have passed since the first enrolled participant at the site. Network injection partners (including replacement partners) will be followed until the corresponding index participant reaches his/her Exit visit.
Treatment Regime
Index participants will be randomized to one of two study arms at a ratio of 1:3 (intervention: standard of care). Index participants in the intervention arm will receive (in addition to the standard harm reduction package) an integrated intervention that includes supported ART regardless of CD4 cell count and facilitated referral for substance use treatment. The psychosocial component of the integrated intervention is designed to improve engagement and retention in HIV care and substance use treatment, and includes systems navigation, counseling to encourage engagement in care and adherence, and social support.
Index participants in the standard of care arm will receive referrals for the in-country standard of care for ART and substance use treatment and a standardized harm reduction package.
Network injection partners in both arms will receive a standardized harm reduction package with referral for substance use treatment, consistent with national guidelines.
Primary Objectives
1) To assess the feasibility of a future randomized controlled trial by:
a) estimating the HIV incidence among network injection partners of index participants in the standard of care arm in three distinct global settings with epidemics driven predominantly by injection drug use; and b) evaluating enrollment and retention of HIV-infected PWID and their HIV-uninfected network injection partners over a period of 12-24 months; and
2) To assess the feasibility, barriers, and uptake of an integrated intervention for prevention of HIV transmission among HIV-infected index participants.
Secondary Objectives
1) To estimate HIV incidence among network injection partners of index participants in the intervention arm.
2) To explore the effect of the integrated intervention, as compared to standard of care, on engagement in HIV care, initiation of ART, retention on ART, ART adherence, and virologic suppression among ART-eligible index participants (index participants meeting national guidelines in the standard of care arm; all index participants in the intervention arm).
3) To explore the effect of the integrated intervention, as compared to standard of care, on the proportion of index participants and network injection partners engaged and retained in substance use treatment.
4) To estimate the size and stability of the injection network of the index participants and how this affects recruitment and retention of network injection partners.
5) To assess the social harms and benefits of research participation for PWID; and
6) If feasible and if funding is identified, to use phylogenetic methods to characterize transmission dynamics in the study cohort.
Other Objectives
Study Documents
HPTN 074 Version 2.0
HPTN 074 Version 1.0
- HPTN 074 Protocol V1.0 - 26 February 2014
- Letter of Amendment #2 - 28 Sept 2016
- Letter of Amendment #1 - 10 Sept 2015
- Clarification Memo #2 - 20 Apr 2015
- Clarification Memo #1 - 17 Mar 2015
Presentations
2017 Annual Meeting
- HPTN 074 Ukraine Site Progress
- Indonesia Site: Progress Report
- Vietnam Site: Enrollment and Retention
- HPTN 074 Publications and closeout
Press
- August 2018: HPTN 074 Demonstrates Significant Benefits Among People Living with HIV Who Inject Drugs
More Study Documents
You must request access to private documents (i.e. SSPs) in Microsoft Teams. You can request access to the Team by emailing Jeff Webb (jwebb@fhi360.org).
You will need a Microsoft account to log in to Team. Click here for instructions on setting up your Microsoft account and accessing the Teams platform.